The GammaPod™ system transforms single fraction stereotactic radiotherapy, as showed by Dr. Marco Trovò – Director of the Complex Structure of Oncological Radiotherapy in Udine. This innovation was introduced through initial data presented as a preview during the annual European Society for Radiotherapy and Oncology.
During the annual ESTRO congress held in Vienna from 12th to 16th May, Dr. Marco Trovò – Director of the Complex Structure of Oncological Radiotherapy in Udine – shared data from the clinical trial* of the first 40 patients who underwent single fraction stereotactic radiotherapy for partial breast irradiation.
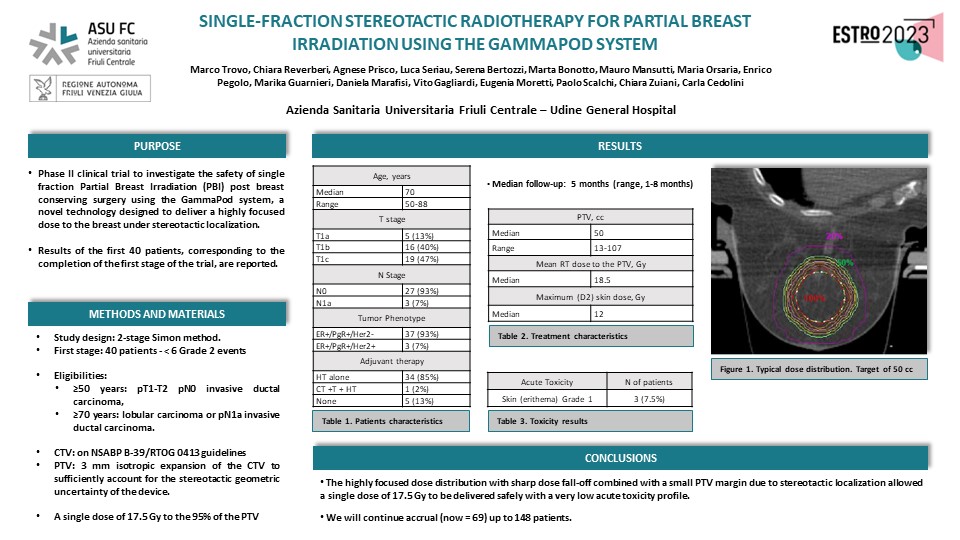
The purpose of this study was to examine the toxicity of partial breast irradiation in a single fraction, following conservative surgery, using the GammaPod™ system – an innovative technology designed to deliver a highly focal dose under stereotactic localization.
This study, called 2-stage Simon method, aims at evaluating the efficiency of a new treatment through two phases. Dr. Trovò explained that, in order to move on to phase two, the first 40 patients must not experience more than six grade 2 events.
The study consists in two groups of patients: the first one, which complies with the ESTRO guidelines; and the second one, in which the patients need to be more than 70 years old – lobular carcinoma or pN1a invasive ductal carcinoma can also be included.
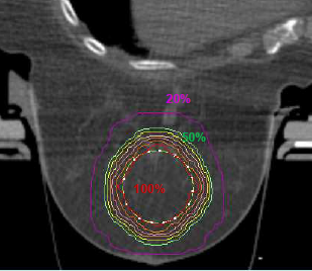
The Planning Target Volume (PTV), such as the geometric volume for delivering the prescribed dose, is obtained by a 3-mm margin expansion over the Clinical Target Volume (CTV), which is the tumor area requiring treatment. The prescribed dose is 17.5 Gy (gray) to the 95% of the PTV.
Over a 15-month follow-up, only three patients experienced a grade 1 skin erythema. These encouraging results were achieved thanks to a maximum dose of 12 Gy and an average PTV size of 50 CC. Stereotactic localization and a highly focused radiation dose are the two elements that allowed a dose of 17.5 Gy to be safely delivered to the PTV in a single fraction, reporting minimal side effects.
With these successful outcomes in hand, the trial can now continue with a larger group of patients (148), in order to provide further confirmation of the safety and efficacy of this innovative technology.
*Special thanks to all the authors of the clinical trial: Marco Trovò, Chiara Reverberi, Agnese Prisco, Luca Seriau, Serena Bertozzi, Marta Bonotto, Mauro Mansutti, Maria Orsaria, Enrico Pegolo, Marika Guarnieri, Daniela Marafisi, Vito Gagliardi, Eugenia Moretti, Paolo Scalchi, Chiara Zuiani, Carla Cedolini.

